Enabling Mass Spectrometry Based Targeted Glycomics Analysis
October 29, 2018
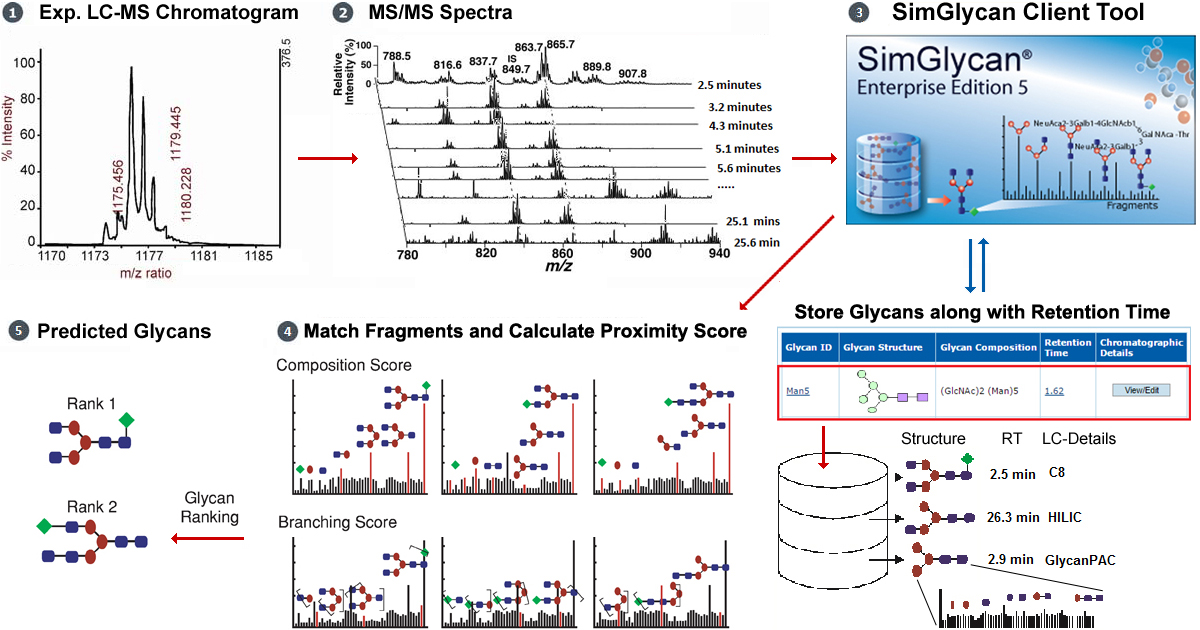
The analysis of N-linked glycans have become increasingly important in therapeutic protein drug development because of their altered bioactivity and efficacy triggered by glycosylation. The analysis protocol involves chromatographic separation of glycans prior to subjecting the sample to mass spectrometry. This reduces sample complexity, minimizes ion suppression, and increases dynamic range and separation of structural isomers. However, accurate identification of glycans in a high throughput manner is hampered by lack of glycan templates. To overcome the challenges, SimGlycan software allows for creating a glycan template, store retention time and other chromatographic details, and identify glycans using retention time as one of the search predicates.
Bovine Fetuin is often used as a test glycoprotein for glycosylation studies in bio-therapeutics. We targeted the glycoproteins (Bovine Fetuin) and extracted N-Linked glycans using PNGaseF enzyme (New England BioLabs). The released glycans were labeled with 2-aminobenzamide (2-AB). A Thermo Scientific™ Dionex™ Ultimate™ 3000 UHPLC instrument with Thermo Scientific™ GlycanPac™ AXR-1 (1.9 μm, 2.1 ×150 mm) column was used to separate the glycans. For structural elucidation, LC-MS2 experiments were conducted using a Thermo Scientific™ Orbitrap Fusion™ Tribrid™ mass spectrometer.
In this attempt to rapidly identify Bovine Fetuin N-Glycans, we created a database consisting of 34 probable unique glycan structures (Table 1). This custom database is used as an MS/MS search template in order to assure correct assignment of glycans.
| 1 | (Gal)2 (GlcNAc)4 (Man)3 | 18 | (Fuc)1 (Gal)2 (GlcNAc)4 (Man)3 |
| 2 | (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)3 | 19 | (GlcNAc)5 (Man)3 |
| 3 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)3 | 20 | (GlcNAc)6 (Man)3 |
| 4 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)4 | 21 | (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)1 |
| 5 | (Gal)4 (GlcNAc)6 (Man)3 (NeuAc)4 | 22 | (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)2 |
| 6 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)5 | 23 | (GlcNAc)2 (Man)6 |
| 7 | (Gal)4 (GlcNAc)6 (Man)3 (NeuAc)5 | 24 | (GlcNAc)2 (Man)8 |
| 8 | (Fuc)1 (Gal)2 (GlcNAc)4 (Man)3 | 25 | (Gal)3 (GlcNAc)5 (Man)3 |
| 9 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)1 | 26 | (GlcNAc)4 (Man)5 |
| 10 | (Fuc)1 (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)2 | 27 | (Gal)4 (GlcNAc)6 (Man)3 |
| 11 | (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)1 (NeuGc)1 | 28 | (Fuc)1 (Gal)2 (GlcNAc)5 (Man)3 |
| 12 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)2 | 29 | (GlcNAc)2 (Man)7 |
| 13 | (GlcNAc)2 (Man)3 | 30 | (Gal)2 (GlcNAc)4 (Man)3 (NeuAc)1 |
| 14 | (GlcNAc)2 (Man)9 | 31 | (Fuc)1 (GlcNAc)5 (Man)3 |
| 15 | (GlcNAc)2 (Man)5 | 32 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)3 |
| 16 | (GlcNAc)4 (Man)3 | 33 | (Gal)3 (GlcNAc)5 (Man)3 (NeuAc)4 |
| 17 | (Fuc)1 (GlcNAc)4 (Man)3 | 34 | (Gal)1 (GlcNAc)3 (Man)3 |
The LC-MS data so generated was processed by SimGlycan for peak detection, peak picking, molecular feature finding, and cluster MS/MS data to LC compounds. The MS/MS data was subjected to the custom database search (consisting of 34 unique glycan structures) using 10 ppm tolerance for precursor and product ions. The database search annotated 55 LC-compounds with 13 unique composition structures.
For detailed workflow, Please Visit: http://premierbiosoft.com/citations/glycomics-posters-technical-application-notes.html
| Comment | Share |
|


