References >> Multiplex PCR
Multiplex PCRIntroduction of Multiplex PCR
Multiplex PCR is a widespread molecular biology technique for amplification of multiple targets in a single PCR experiment. In a multiplexing assay, more than one target sequence can be amplified by using multiple primer pairs in a reaction mixture. As an extension to the practical use of PCR, this technique has the potential to produce considerable savings in time and effort within the laboratory without compromising on the utility of the experiment.
Types of Multiplex PCR
Multiplexing reactions can be broadly divided in two categories:
1. Single Template PCR Reaction
This technique uses a single template which can be a genomic DNA along with several pairs of forward and reverse primers to amplify specific regions within a template.
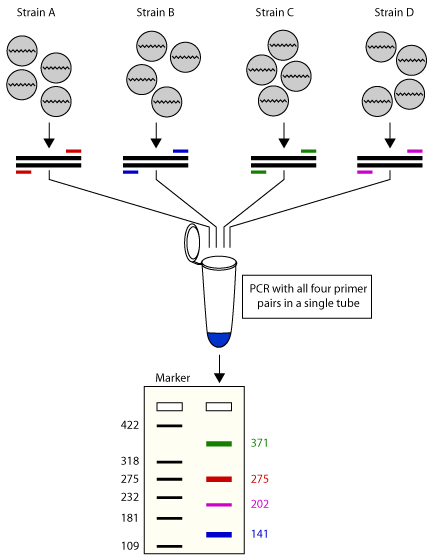
2. Multiple Template PCR Reaction
It uses multiple templates and several primer sets in the same reaction tube. Presence of multiple primers may lead to cross hybridization with each other and the possibility of mis-priming with other templates.
Primer Design Parameters for Multiplex PCR
Design of specific primer sets is essential for a successful multiplex reaction. The important primer design considerations described below are a key to specific amplification with high yield.
1. Primer Length
Multiplex PCR assays involve designing of large number of primers, hence it is required that the designed primer should be of appropriate length. Usually, primers of short length, in the range of 18-22 bases are used.
2. Melting Temperature
Primers with similar Tm, preferably between 55°C-60°C are used. For sequences with high GC content, primers with a higher Tm (preferably 75°C-80°C) are recommended. A Tm variation of between 3°-5° C is acceptable for primers used in a pool.
3. Specificity
It is important to consider the specificity of designed primers to the target sequences, while preparing a multiplex assay, especially since competition exists when multiple target sequences are in a single reaction vessel.
4. Avoid Primer Dimer Formation
The designed primers should be checked for formation of primer dimers, with all the primers present in the reaction mixture. Dimerization leads to unspecific amplification.
All other parameters are similar to standard PCR primer design guidelines.
Advantages of Multiplex PCR
1. Internal Controls
Potential problems in a simple PCR include false negatives due to reaction failure or false positives due to contamination. False negatives are often revealed in multiplex assays because each amplicon provides an internal control for the other amplified fragments.
2. Efficiency
The expense of reagents and preparation time is less in multiplex PCR than in systems where several tubes of uniplex PCRs are used. A multiplex reaction is ideal for conserving costly polymerase and templates in short supply.
3. Indication of Template Quality
The quality of the template may be determined more effectively in multiplex than in a simple PCR reaction.
4. Indication of Template Quantity
The exponential amplification and internal standards of multiplex PCR can be used to assess the amount of a particular template in a sample. To quantitate templates accurately by multiplex PCR, the amount of reference template, the number of reaction cycles, and the minimum inhibition of the theoretical doubling of product for each cycle must be accounted.
Applications of Multiplex PCR
-
Pathogen Identification
-
High Throughput SNP Genotyping
-
Mutation Analysis
-
Gene Deletion Analysis
-
Template Quantitation
-
Linkage Analysis
-
RNA Detection
-
Forensic Studies
Multiplex PCR Primer Design with PrimerPlex
PrimerPlex is an efficient tool to design specific oligos for multiplex PCR assays.
The program checks the oligos for cross reactivity and minimizes Tm mismatches to give you the best possible multiplex set. In this process, it analyzes millions of possible multiplex sets in an a few seconds and presents a list of alternate sets.
Design Real time Multiplex PCR Primers using Beacon Designer and AlleleID®



