Protein Glycosylation
The addition of a carbohydrate moiety to a protein molecule is referred to as protein glycosylation. It is a common post translational modification for protein molecules involved in cell membrane formation. During this process,the linking of monosaccharide units to the amino acid chains sets up the stage for a series of enzymatic reactions that lead to the formation of glycoproteins (n and o linked oligosaccharides that are found to a protein entity). In all 16 known enzymes are supposed to mediate this reaction. A typical glycoprotein has at least 41 bonds which involve 8 amino acids and 13 different monosaccharide units and includes the glycophosphatidylinositol (GPI) and phosphoglycosyl linkages. Protein glycosylation helps in proper folding of proteins, stability and in cell to cell adhesion commonly needed by cells of the immune system. The major sites of protein glycosylation in the body are ER, Golgi body, nucleus and the cell fluid.
Protein glycosylation can be categorized in two main types:
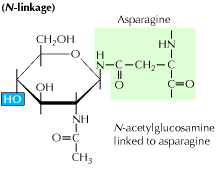 a) N-linked glycosylation: It begins with the addition of a 14-sugar precursor to an asparagine amino acid. It contains glucose, mannose and n-acetylglucosamine molecules. This entity is then transferred to the ER lumen. The oligosaccharyl transferase enzyme attaches the oligosaccharide chain to asparagine that occurs in the tripeptide sequence, Asn-X-Ser or Asn-X-Thr. X can be any amino acid other than Proline. The oligosaccharide attached protein sequence now folds correctly and is now translocated to the Golgi body where the mannose residue is removed.
a) N-linked glycosylation: It begins with the addition of a 14-sugar precursor to an asparagine amino acid. It contains glucose, mannose and n-acetylglucosamine molecules. This entity is then transferred to the ER lumen. The oligosaccharyl transferase enzyme attaches the oligosaccharide chain to asparagine that occurs in the tripeptide sequence, Asn-X-Ser or Asn-X-Thr. X can be any amino acid other than Proline. The oligosaccharide attached protein sequence now folds correctly and is now translocated to the Golgi body where the mannose residue is removed.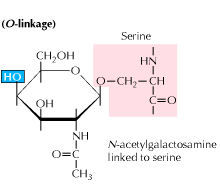
b) O-linked glycosylation: Glycosylation begins with an enzyme mediated addition of N-acetyl-galactosamine followed by other carbohydrates to serine or threonine residues. Studies reveal that O linked glycosylation occurs at a later stage in protein processing.


